Cyclohexylmethanol From Grignard Reagent
Give structures of the products you would expect when each of the following alcohol reacts with a HCl ZnCl 2 b HBr and c SOCl 2. Write structures of the products of the following reactions.
2-methyl propan-1-ol can be prepared using grignard reagent.

. In chemistry an alcohol is a type of organic compound that carries at least one hydroxyl functional group OH bound to a saturated carbon atom. In commercial applications the alkylating agents are generally alkenesProtonation of alkenes generates carbocationsA laboratory-scale example by the. The term alcohol originally referred to the primary alcohol ethanol ethyl alcohol which is used as a drug and is the main alcohol present in alcoholic drinksAn important class of alcohols of which methanol and ethanol are.
The Grignard reaction is an organometallic chemical process in which a carbonyl group in an aldehyde or ketone is substituted with alkyl allyl vinyl or aryl-magnesium halides Grignard reagent. The creation of carboncarbon bonds is dependent on this process. Alkenes as alkylating agents.
Show how are the following alcohols prepared by the reaction of a suitable Grignard reagent on methanal. For primary and possibly secondary alkyl halides a carbocation-like complex with the Lewis acid R ---X---MX n is more likely to be involved rather than a free carbocation.
How To Convert Cyclohexanol To Cyclohexylmethanol Quora
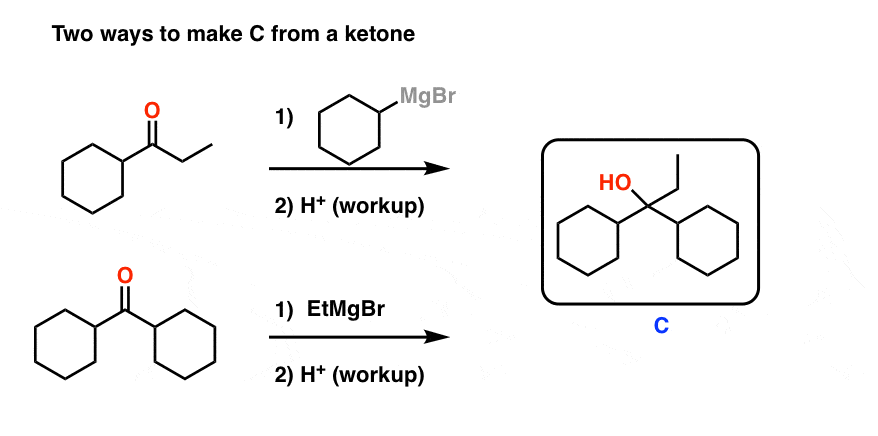
Grignard Practice Problems Synthesis 1 Master Organic Chemistry

Name The Reagents Used In The Following Reactions I Oxidation Of A Primary Alcohol To Carboxylic Acid Ii Oxidation Of A Primary Alcohol To Aldehyde Iii Bromination Of Phenol To 2 4 6 Tribromophenol Iv Benzyl Alcohol

Show How Will You Synthesise I 1 Phenylethanol From A Suitable Alkene Ii Cyclohexylmethanol Using An Alkyl Halide By An Sn2 Reaction Iii Pentan 1 Ol Using A Suitable Alkyl Halide

No comments for "Cyclohexylmethanol From Grignard Reagent"
Post a Comment